ISO 13485
ISO 13485 is an international standard that manufacturers, suppliers, and distributors of medical devices must comply with in order to demonstrate that they are adhering to the legal requirements regarding medical devices. This standard can also be applied to other companies involved in the chain of a medical device. This includes design and development, storage, handling, distribution, maintenance, installation, service, and cleaning/sterilization of medical devices.
Customer Satisfaction Survey
In 2021 and 2022, Life & Mobility conducted a customer satisfaction survey among various customer groups. With a response rate of 17.3%, we are very satisfied and proud of an average satisfaction score of 8.4. In the questionnaire, we asked our customers about their experiences regarding our products, services, and collaboration, among other things.
An average customer satisfaction score of 8.4!
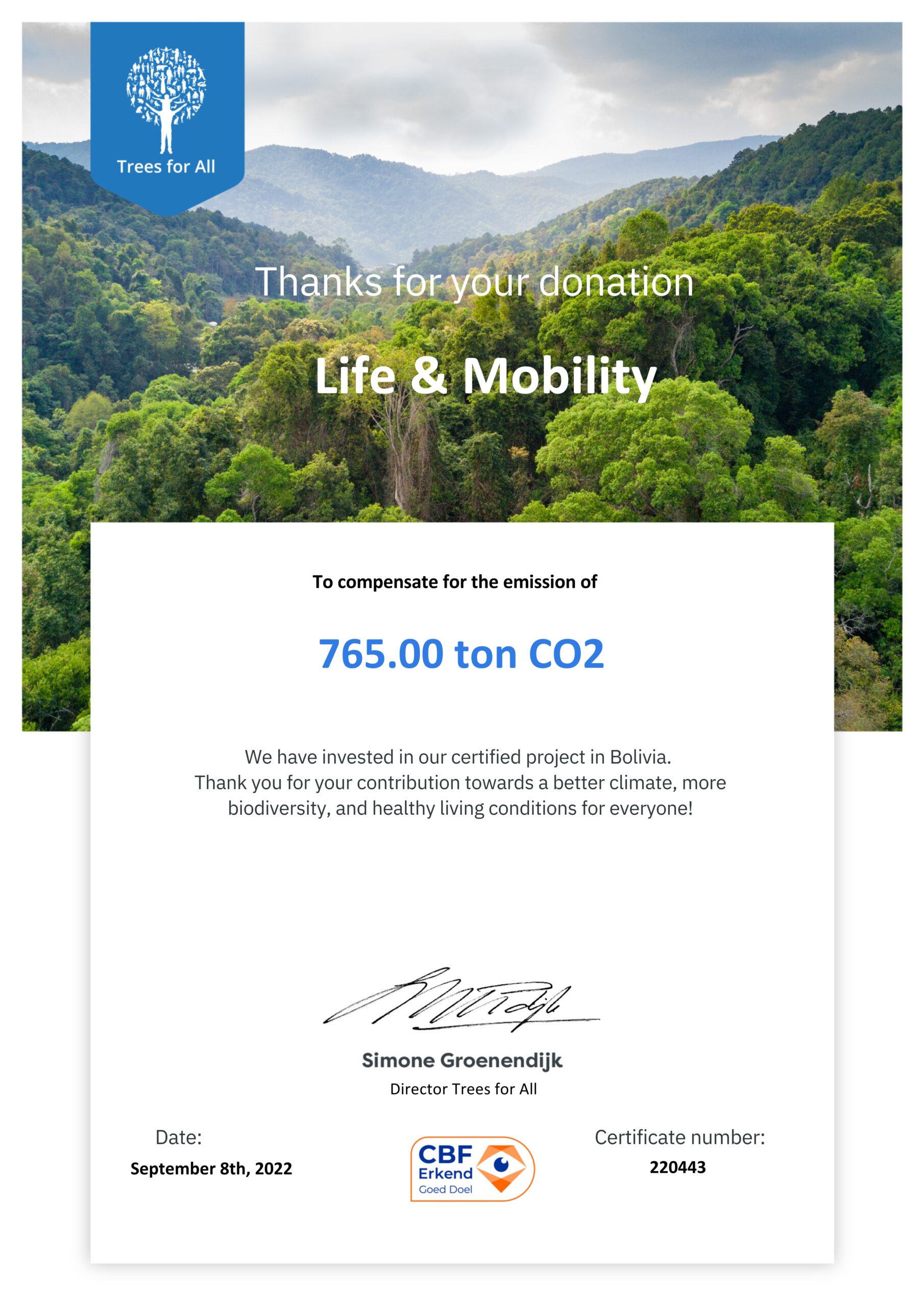
CO2- neutral certification
For a livable earth with a healthy climate, it is important that we reduce as much CO2 as possible. The CO2 emissions that cannot (yet) be avoided are compensated for in a sustainable manner by planting trees in certified forest projects.
NKH (National Quality Mark for Assistive Devices)
In the Nettherlands, the National Quality Mark for Assistive Devices helps in choosing a high-quality assistive device. This ensures that the purchaser knows that the company where this device is purchased meets all strict quality requirements. Entrepreneurs with the National Quality Mark for Assistive Devices are guaranteed to be knowledgeable, reliable, and transparent. More and more organizations within this industry have the quality mark, serving a total of 80% of the market in the Netherlands.
The 7 assurances of the National Quality Mark for Assistive Devices:
- You will receive expert advice from professionals who have been carefully trained in advising, manufacturing and adapting assistive devices;
- You will receive objective information about the possibilities for obtaining an assistive device;
- You will receive a safe assistive device that demonstrably meets the relevant European safety requirements;
- You will receive a good, clear explanation of how to use the assistive device optimally and safely in your specific situation;
- You will receive guaranteed aftercare and service in case something goes wrong with the assistive device;
- You will receive the assurance of a good complaints procedure;
- You can be sure that an accredited company pays attention to Corporate Social Responsibility.